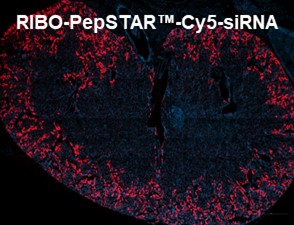
Ribo is Advancing Kidney-Targeted Therapeutics
We recently presented a poster at the ASN Kidney Week in Texas, showcasing the kidney-targeted delivery of siRNA using our RIBO-PepSTAR™
Read our latest news and insights below.

We recently presented a poster at the ASN Kidney Week in Texas, showcasing the kidney-targeted delivery of siRNA using our RIBO-PepSTAR™
BEIJING, China & GOTHENBURG, Sweden — 2025-10-24 — Suzhou Ribo Life Science Co., Ltd. and Ribocure Pharmaceuticals AB (Ribo), today

SuZhou Ribo life science Ltd. Co. and Ribocure Pharmaceuticals AB (Ribo) presented four key scientific findings at the 2025 European

Mölndal, July 2nd 2025 — We are delighted to announce that distinguished Swedish investors Erik Selin and Jacob Torell have

Ribocure Pharmaceuticals AB and Suzhou Ribo Life Science Ltd., “Ribo”, is pleased to announce the successful completion of patient recruitment

Ribocure Pharmaceuticals AB and Medtanken Group AB today present a strategic collaboration that allows clinical research to be applied directly

Pheiron and Ribo are excited to announce that the first two strategic collaboration milestones have been successfully achieved, marking a

Today marks an important and exciting milestone for Ribo as we strive to improve treatments for rare diseases. In conjunction

Recent news highlights that the Factor XI inhibitor space is emerging as the next significant advancement in cardiovascular care, and

RBD4059 is a GalNAc-conjugated small-interfering RNA (siRNA) drug independently developed by Suzhou Ribo Life Science. Ltd (Ribo) based on the

Suzhou Ribo Life Science and Ribocure AB are excited to share that we have achieved a key pre-clinical milestone in

On October 28, 2024, Ribocure Pharmaceuticals AB and Suzhou Ribo Life Science Ltd (Ribo) received authorization from the Swedish Medicinal

With the ambition to become a world-leading clinical oligonucleotide research company, Ribocure Pharmaceuticals AB has established Ribocure Clinic, to focus

Ribocure Pharmaceuticals AB and Suzhou Ribo Life Science Ltd (Ribo) have entered a strategic partnership with Pheiron to leverage its

On September 10, 2024, Ribocure Pharmaceuticals AB and Suzhou Ribo Life Science Ltd (Ribo) received authorization from the Swedish Medicinal

We thank the European Society of Cardiology #ESC Congress, session chairs, and all participants in the session for the opportunity

On May 27, 2024, Ribocure Pharmaceuticals AB and Suzhou Ribo Life Science Ltd (Ribo) received authorization from the Swedish Medicinal

Today we highlight the Clinical Trials Day and the importance of clinical research at Ribocure Pharmaceuticals and Ribocure Clinic. Ribocure

Ribocure Pharmaceuticals/Suzhou Ribo Life Science Co. Ltd. (“Ribo”) announce Notice of Allowance issued respectively by US Patent Office and European
On April 10, 2024, Ribo and Ribocure announce the completion of enrolment and dosing of the Ph1 clinical trial with

Ribocure Pharmaceuticals AB announces EMA authorisation to proceed with a Phase II clinical trial in Sweden. The trial will evaluate

Ribocure Pharmaceuticals AB is a clinical stage biotech company developing oligonucleotide-based therapeutics (siRNA). Across the globe, chronic kidney disease (CKD) affects

Ribocure Pharmaceuticals AB is a clinical stage biotech company developing oligonucleotide-based therapeutics (siRNA). Our primary focus lies in addressing unmet

Mölndal, Sweden, Kunshan, China and Ingelheim, Germany – 3 January 2024 Suzhou Ribo Life Science Co. Ltd. and Ribocure Pharmaceuticals

Our CEO has been portrayed in GoCo news. Here he shared his views on how productive collaboration between industry, academia

With the ambition to become the leading clinical oligonucleotide research company in Sweden, Ribocure Pharmaceuticals AB established Ribocure Clinic, with

John Taylor has joined Ribocure Pharmaceuticals AB as Vice President and Head of Global Business Development. John will lead the

Suzhou Ribo Life Science (Ribo) and Ribocure Pharmaceuticals (Ribocure) presented new data from three projects from its cardiovascular pipeline at

Thanks EASL for a great meeting and also the opportunity to simultaneously show data from our HBV program, both with

We arranged our inauguration ceremony together with both friends and colleagues from academia and industry, as well as many persons

Li-ming Gan, CEO at Ribocure Pharmaceuticals, is for the first time showing our preclinical PoC data at the EASL meeting

Ribocure Pharmaceuticals AB receives Authorisation for a Phase II clinical trial to evaluate efficacy and safety of its novel siRNA

In June we move into the entire fifth floor of the GoCo Clinic building and established a unique “from bench-to-bed”

GoCo House Lunch Launch took place and we were there from Ribocure Pharmaceuticals to meet our future neighbours and potential

World’s first siRNA-based FXI silencing agent enters clinical development – an important step toward a new generation of anti-thrombotic therapies

Ribocure participated in The Innovative Ecosystem at GoCo Arena event and pitched our vision and aims in just 3 minutes.

Visit at GoCo and Gothenburg by one of the founders, Dr Hongyan Zhang, from the parent comany. Impressed and inspired

First tour at the future office. A near 1000 sqm facility with R&D, lab and clinical trail unit will be

Ribocure Pharmaceuticals AB establishes in GoCo Clinic- putting Sweden in the spotlight within oligonucleotide therapeutics GoCo Health Innovation City can
Ribocure will be expanding heavily in Sweden and looking for new talents to join, and academic associations as well as small and large enterprise partners to collaborate with, with the joint goal of developing highly differentiated and innovative oligonucleotide therapeutics to help patients in need.

Sitemap
About Ribocure
Contact us